Highly Validated Approaches to Highly Unmet Needs.
Developing Treatments for Severe Liver Disease.
about
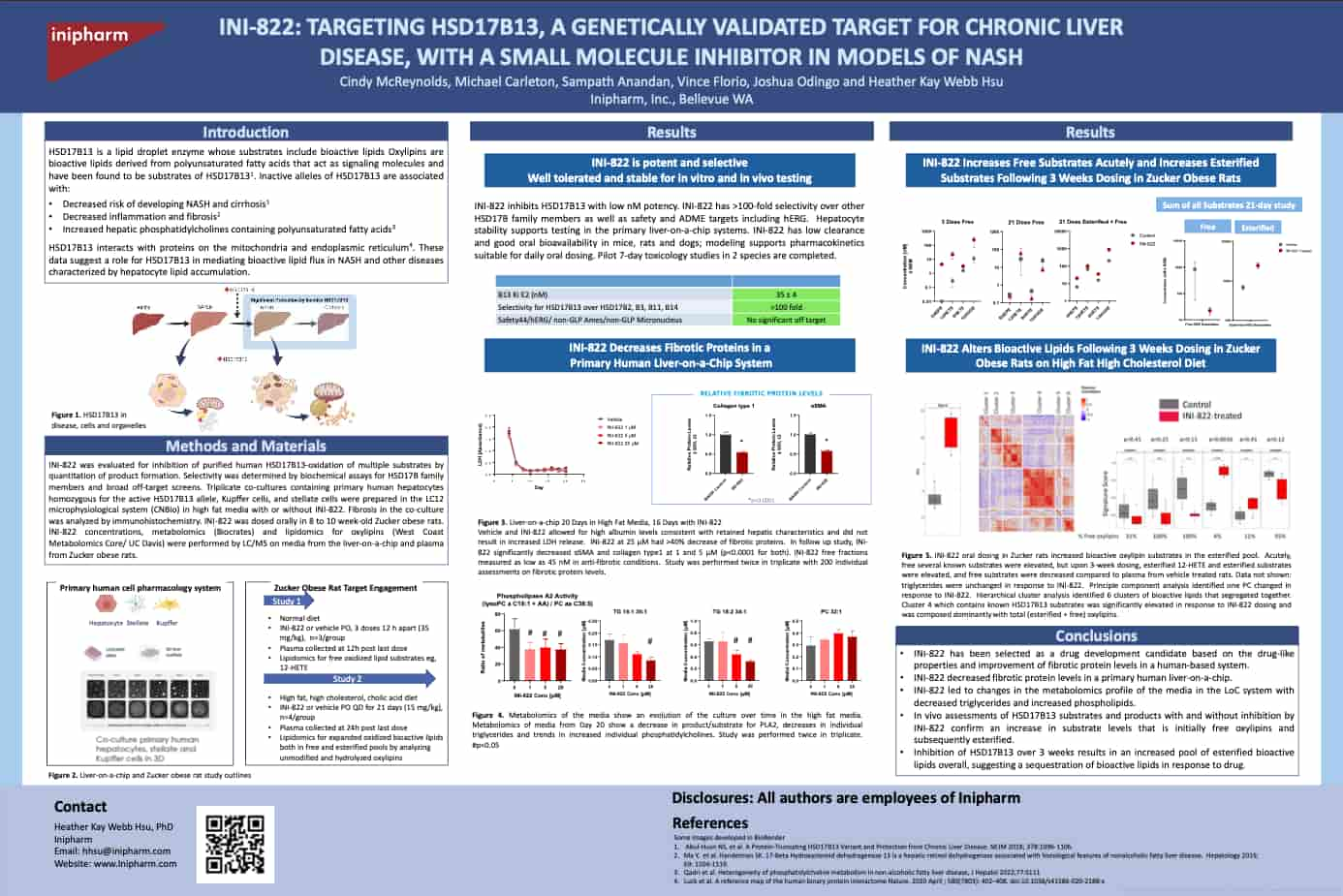
Inipharm is a clinical-stage biopharmaceutical company focused on discovering and developing therapies for severe liver diseases. Inipharm's lead program is focused on the highly validated, genetically-defined target, HSD17B13.
Inipharm is conducting a Phase 1 clinical trial, including MASH patients, of INI-822, a first in class small molecule inhibitor of HSD17B13.
There is extensive, consistent evidence that genetic variants of HSD17B13 are associated with significantly lower rates and severity of multiple liver diseases. Building on these novel insights into the biologic activity of HSD17B13, Inipharm is advancing a pipeline of small-molecule therapies that target the activity of this protein.
our science
Inipharm is developing small-molecule inhibitors of HSD17B13 to treat fibrotic liver diseases. Inipharm's inhibitors have been shown to drive alterations in lipid metabolism and improvements in liver enzymes that are consistent with changes seen with the protective variant in humans. In human liver cell-based systems, INI-822 demonstrated anti-fibrotic effects.
Protective variants in HSD17B13 are associated with reduced risk incidence and/or the severity of numerous liver diseases.
- HSD17B13 has been identified as a novel target for NASH and driver of liver disease from a genome wide association study.
- Loss of function variants in HSD17B13 are associated with reduced risk incidence and/or the severity of numerous liver diseases.
- Importantly, HSD17B13 has been shown to be involved in pathways associated with lipotoxicity, inflammation, and fibrosis.

INI-822, development candidate
- Reduced fibrotic markers in human liver cell model
- Improved liver enzymes in multiple in vivo models
- Replicated lipidomic changes seen with protective HSD17B13 variant
leadership

Brian Farmer
Co-Founder, Chief Executive Officer
Brian has over 25 years of experience in the healthcare industry and has held senior leadership positions in several pharmaceutical companies, including as Chief Business Officer and Chief Financial Officer at Cirius Therapeutics. He previously co-founded and served as Chief Business Officer of Mavupharma, until its acquisition by AbbVie in 2018, and served as Chief Business Officer at Laguna Pharmaceuticals and as Vice President of Corporate Development and Operations at Sequel Pharmaceuticals. Prior to Sequel, he was responsible for corporate development at NovaCardia until its acquisition by Merck. Before this, he was a global marketing associate at Eli Lilly and worked in marketing and sales operations for Prudential HealthCare and in public health program management. Brian is also an Entrepreneur-in-Residence at Frazier Healthcare Partners. He holds an MBA from the University of Texas and his BS from Trinity University.

Heather Hsu, PhD
Co-Founder, Chief Scientific Officer
Heather has over 23 years of pharmaceutical/biotech industry experience with research and development. Her PhD in Biological Chemistry from the University of Michigan included a collaboration with Eli Lilly. In the years since, Heather has led pharmacology and nonclinical research teams in indication areas, including cardiovascular and liver diseases, at companies including Johnson & Johnson and Gilead Sciences, as well as Calistoga Pharmaceuticals. Her work includes supporting 26 clinical trials with design and clinical pharmacology analysis and regulatory filings including 17 INDs and an NDA.

Chuhan Chung, MD
Chief Medical Officer
Chuhan Chung MD serves as Chief Medical Officer at Inipharm. Dr. Chung most recently was Executive Director/Global Development Lead at Gilead Sciences, Inc. There, he worked on development of the acetyl-coA carboxylase inhibitor, GS-0976, through Phase 2 studies. He was also the clinical lead for the Phase 3 study of primary sclerosing cholangitis. Previously, he was Associate Professor at the Yale University School of Medicine, Section of Gastroenterology & Hepatology and maintained an independently funded laboratory for 13 years. He earned a bachelor’s degree from Wesleyan University, a medical degree from Medical College of Pennsylvania, and completed internship, residency, and a hepatology fellowship at Feinberg School of Medicine at Northwestern University. He is an author on over 50 peer-reviewed articles.

Sampath Anandan, PhD
VP, Discovery
Sampath has over 22 years of drug discovery experience as a medicinal chemist and has held team leadership positions in several biotech, pharmaceutical companies. Most recently he served as an associate director of chemistry and project team leader at Protagonist Therapeutics. Prior to that he was a chemistry team lead at Novartis, served as an associate director at Arete Therapeutics, group leader at Miikana (acquired by Entramed) and as a research fellow at Vicuron Pharmaceuticals (acquired by Pfizer). Throughout his career he has successfully co-lead oncology, anti-viral, anti-bacterial, metabolic, and GI disease drug discovery programs from hit to IND nominations. Sampath obtained his PhD in organic chemistry from the Indian Institute of Science, Bangalore, India.

Katelyn Paterson
Senior Director, Clinical Operations
Katelyn has over 15 years of experience in the Clinical Research space, including clinical trial management roles in both the pharmaceutical and medical device spaces. Her work has spanned Phase I-III and Post Market studies across 5 continents. She holds a BS from the University of Rochester.
board of directors
Bob Baltera
Cirius Therapeutics CEO
Bob is a seasoned pharmaceutical executive with broad business and product management experience.
He is the Chief Executive Officer of Cirius Therapeutics and an Entrepreneur-in-Residence with Frazier Healthcare Partners. Bob previously served as Chief Executive Officer of Laguna Pharmaceuticals and Chief Executive Officer of Amira Pharmaceuticals until its $475 million acquisition by Bristol-Myers Squibb in 2011. Prior to his tenure at Amira, Bob held a number of senior management positions over 17 years at Amgen. Bob received his MBA from the Anderson School at the University of California, Los Angeles, and an MS in genetics and a BS in microbiology from The Pennsylvania State University.
Hannah Chang, MD, PhD
Managing Director, Platanus Capital
Hannah Chang is a Managing Director at Platanus Capital. Dr. Chang focuses on early-stage biopharmaceutical venture capital investments out of the firm's San Francisco office. Representative investments include Semma Therapeutics (acquired by Vertex Pharmaceuticals), Akouos (NASDAQ: AKUS), Korro Bio, Gemini Therapeutics, and PACT Pharma.
She was previously an investment professional at 5AM Ventures where she helped found IDEAYA Biosciences (NASDAQ: IDYA) and served as a Board Observer for Crinetics Pharmaceuticals (NASDAQ: CRNX), among others. Earlier in her career Dr. Chang was a Project Leader at The Boston Consulting and a member of the Healthcare Practice.
Dr. Chang obtained her MD from Harvard Medical School, PhD in Biophysics from Harvard University, and BA in Chemistry from Princeton University. She trained clinically in internal medicine and ophthalmology at the Beth Israel Deaconess Medical Center and Massachusetts Eye and Ear Infirmary, respectively. She is a licensed physician in the District of Columbia.
Brian Daniels, MD
Partner, 5AM Ventures
Brian joined 5AM Ventures as a Venture Partner in 2014 and transitioned to Partner in 2018. Dr. Daniels previously spent over two decades in clinical drug development, including leading the Development and Medical Affairs at Bristol-Myers Squibb for the last ten years. He directed the development of numerous innovative medicines that have contributed to the improvement for patients across a range of serious diseases. These include: ORENCIA and NULOJIX in immunology/transplant, REYATAZ, DAKLINZA and BARACLUDE in virology, ONGLYZA, FARXIGA and MYALEPT in metabolics, ELIQUIS in CV and YERVOY, OPDIVO, SPRYCEL and IXEMPRA in oncology. Dr. Daniels is currently a Director at these 5AM companies: Spyryx Bioscience, Nohla Therapeutics, Rarecyte, and Ideaya Biosciences (independent). He leads the Clinical Advisory Board for Aprea Therapeutics and also served on the Scientific Advisory Board of Novira. Dr. Daniels received BS and MS degrees from MIT and his MD from Washington University in St. Louis. He trained in internal medicine at New York Hospital and Rheumatology/Immunology at UCSF.
Dan Estes, PhD
Frazier Healthcare Partners
Dan is a General Partner on the Frazier Life Sciences team and has been involved with investing in and building many of Frazier's biopharmaceutical portfolio companies over the past 9 years, focusing on investments in both development-stage and commercial-stage pharmaceutical companies. He has led Frazier's investments in, and serves on the boards of, Arcutis (NASDAQ: ARQT, co-founder) and Cirius Therapeutics. He previously served on the board of Semnur Pharmaceuticals (acquired by Scilex).
Dan has also been involved in Frazier's investments in Acerta Pharma BV (acquired by AstraZeneca), Tobira Therapeutics (acquired by Allergan), Ignyta (acquired by Roche), ARMO (acquired by Lilly), Iovance (NASDAQ: IOVA), and PreCision Dermatology (acquired by Valeant).
Prior to joining Frazier, Dan was a management consultant with McKinsey & Company's healthcare practice, where he led teams that advised leading global pharmaceutical and biotechnology companies on R&D strategy and business development strategy. He received his PhD in Biomedical Engineering from the University of Michigan and holds a BS in Electrical Engineering from Stanford University, where he is a member of Tau Beta Pi.
In 2018, Dan was named a “40-and-under Silicon Valley biotech investing star” by Business Insider.
Brian Farmer
Co-Founder, Chief Executive Officer
Brian has over 25 years of experience in the healthcare industry and has held senior leadership positions in several pharmaceutical companies, including as Chief Business Officer and Chief Financial Officer at Cirius Therapeutics. He previously co-founded and served as Chief Business Officer of Mavupharma, until its acquisition by AbbVie in 2018, and served as Chief Business Officer at Laguna Pharmaceuticals and as Vice President of Corporate Development and Operations at Sequel Pharmaceuticals. Prior to Sequel, he was responsible for corporate development at NovaCardia until its acquisition by Merck. Before this, he was a global marketing associate at Eli Lilly and worked in marketing and sales operations for Prudential HealthCare and in public health program management. Brian is also an Entrepreneur-in-Residence at Frazier Healthcare Partners. He holds an MBA from the University of Texas and his BS from Trinity University.
Michael Gallatin, PhD
Co-Founder, SAB Chair
Mike has over 35 years of experience as a scientist and executive in the biopharma industry and is a Senior Advisor on the Frazier Life Sciences team. He has co-founded multiple companies, including Mavupharma (acquired by AbbVie), Calistoga Pharmaceuticals (acquired by Gilead Sciences), and Stromedix (acquired by Biogen Idec). Mike served as President of Calistoga, which was the first company to demonstrate the clinical benefit of an isoform selective PI3K (idelalisib) inhibitor in hematologic malignancies. Previously, Mike was one of the founding scientists at ICOS Corporation, where he served as VP and Scientific Director. Mike's responsibilities at ICOS included discovery, preclinical research, medicinal chemistry, and process chemistry groups including those that helped generate and support the worldwide registration and launch of Cialis. Earlier, Mike developed expertise in the fields of immunology/inflammation and oncology while on the faculty at the Fred Hutchinson Cancer Center. Mike has also been a member of the Scientific Advisory Boards of the Keystone Symposia, Caprion, and the University of Texas Department of Chemistry. Mike received his PhD from the University of Alberta Department of Immunology.
Craig Gibbs, PhD, MBA
Chief Executive Officer, Asher Biotherapeutics Inc.
Craig Gibbs, PhD, MBA has been Chief Executive Officer of Asher Biotherapeutics Inc. since September of 2020 and also serves on the Boards of Directors of Aridis Pharmaceuticals Inc. and Inipharm Inc. and the Board of Trustees for the Leukemia and Lymphoma Society. From September 2015 until April 2020, Dr. Gibbs was the Chief Business Officer at Forty Seven Inc. and served on the Board of Directors of Tobira Therapeutics from May 2013 to September 2016. From 1992 to 2013, Dr. Gibbs worked for Gilead Sciences in a variety of leadership positions spanning Biology Research, Corporate Development and, most recently, Vice President of Commercial Strategy/Commercial Planning and Operations. Prior to Gilead, Dr. Gibbs served as a Scientist in the Department of Protein Engineering at Genentech, Inc. He received his BSc in Biochemistry from Massey University and his PhD in Molecular Biology from the University of Glasgow in Scotland and his MBA from Golden Gate University.
scientific advisory board
Jim Bristol, PhD
Former Head of Discovery, Pfizer
Jim has over 40 years of experience as a chemist and leader of pharmaceutical discovery and research divisions and is a Senior Advisor on the Frazier Life Sciences team.
Jim spent over 30 years in drug discovery research and development at Schering-Plough, Parke-Davis, and Pfizer. He most recently held the position of Senior Vice President, Worldwide Discovery Research at Pfizer and was responsible for research activities in the United States, United Kingdom, and Japan, involving over 3,000 scientists at 7 research laboratory sites. Jim has been involved with the discovery and development of many drugs that have been FDA approved or are currently in clinical development, including Lipitor, Lyrica, and Ibrance.
Jim has also been an Adjunct Professor of Medicinal Chemistry at the University of Michigan, and Editor-in-Chief of the American Chemistry Society (ACS) publication series called Annual Reports in Medicinal Chemistry. Jim's research in drug discovery programs – related to the treatment of gastrointestinal, central nervous system, and cardiovascular diseases with a focus on mechanistic biochemistry – have been recorded in over 100 publications, abstracts, and patents.
Jim received a PhD in Organic Chemistry from the University of New Hampshire and a BS in Chemistry from Bates College. He was an NIH Postdoctoral Research Fellow at the University of Michigan and a postdoctoral Fellow at The Squibb Institute for Medical Research.
Jerry Colca, PhD
CSO and Co-Founder, Cirius
Jerry is a widely recognized expert in the pathophysiology of insulin resistance and the pharmacological mechanisms of action of insulin sensitizing drugs. He has dedicated his career to studying the endocrine control of metabolism as it relates to diabetes and has been focused on insulin sensitizers from the early days of their discovery.
Jerry started his career in industry at The Upjohn Company where he studied the mechanism of action of the thiazolidinediones and served as project leader for the selection and early development of pioglitazone hydrochloride (Actos®). Jerry continued his metabolic disease research with the company through its mergers with Pharmacia, Monsanto-Searle and Pfizer. In 2006 Dr. Colca co-founded MSDC, a company focused on development of compounds to treat metabolic diseases based on the discovery of a novel mitochondrial protein complex that is believed to be the mechanism through which insulin sensitizing drugs achieve effective glucose control.
Jerry received his PhD and MS in physiology and biochemistry from the University of Houston and served as a post-doctoral fellow in the department of pathology at Washington University, School of Medicine in St. Louis. Jerry also received his BS, in biology, from the University of Houston.
Michael Gallatin, PhD
Co-Founder, SAB Chair
Mike has over 35 years of experience as a scientist and executive in the biopharma industry and is a Senior Advisor on the Frazier Life Sciences team. He has co-founded multiple companies, including Mavupharma (acquired by AbbVie), Calistoga Pharmaceuticals (acquired by Gilead Sciences), and Stromedix (acquired by Biogen Idec). Mike served as President of Calistoga, which was the first company to demonstrate the clinical benefit of an isoform selective PI3K (idelalisib) inhibitor in hematologic malignancies. Previously, Mike was one of the founding scientists at ICOS Corporation, where he served as VP and Scientific Director. Mike's responsibilities at ICOS included discovery, preclinical research, medicinal chemistry, and process chemistry groups including those that helped generate and support the worldwide registration and launch of Cialis. Earlier, Mike developed expertise in the fields of immunology/inflammation and oncology while on the faculty at the Fred Hutchinson Cancer Center. Mike has also been a member of the Scientific Advisory Boards of the Keystone Symposia, Caprion, and the University of Texas Department of Chemistry. Mike received his PhD from the University of Alberta Department of Immunology.
Kris Kowdley, MD
Director, Liver Institute Northwest
Dr. Kowdley received his BS in Biology and Anthropology as a member of the Dean's List at Columbia University, and his medical degree from Mount Sinai School of Medicine. He completed his internship and residency at Oregon Health Science University and a Fellowship in Gastroenterology and Hepatology at Tufts University School of Medicine.
Dr. Kowdley is internationally recognized as a clinician, educator and researcher in the area of liver disease and has presented his research on liver diseases at more than 150 national and international meetings and scientific symposia. He is the author of more than 400 articles, book chapters, reviews and commentaries in this area and has been published in the Annals of Internal Medicine, Archives of Surgery, Gastroenterology, Hepatology, American Journal of Physiology, New England Journal of Medicine, and among other professional publications.
Dr. Kowdley has extensive experience in clinical trials in all areas of liver disease, ranging from hepatitis C, cholestatic liver disease, PBC, PSC, nonalcoholic steatohepatitis and hepatitis B. He has been a principal investigator in several NIDDK-sponsored clinical trials in primary biliary cirrhosis, primary sclerosing cholangitis and is a member of executive committee of the nonalcoholic steatohepatitis clinical research network (NASH CRN). Dr. Kowdley has also served as the lead investigator of several major international clinical trials in hepatitis C.
Dr. Kowdley laboratory program is focused on the role of iron as a co-factor in many liver diseases, ranging from hepatitis C, hemochromatosis and nonalcoholic steatohepatitis (NASH). He has developed murine models for NASH and is currently exploring the contribution of hepatic iron deposition on the severity of NASH.
Dr. Kowdley's research program has been continuously funded by the NIDDK since 1999 in addition to several grants from foundations and scientific societies
Rohit Loomba, MD
Professor of Medicine, Director of Hepatology, UCSD
Dr. Rohit Loomba is a Professor of Medicine (with tenure), Director of Hepatology, at University of California at San Diego. He is an internationally recognized thought leader in translational research and innovative clinical trial design in nonalcoholic steatohepatitis (NASH), and non-invasive assessment of NAFLD using advanced imaging modalities.
Dr. Loomba is the founding director of the UCSD NAFLD Research Center where his team is conducting cutting edge research in all aspects of NAFLD including non-invasive biomarkers, genetics, epidemiology, clinical trial design, imaging end-points, and integrated OMICs using microbiome, metabolome and lipidome. This integrated approach has led to several innovative applications such as establishment of MRI-PDFF as a non-invasive biomarker of treatment response in early phase trials in NASH, which has now been adopted in more than 25 clinical trials conducted worldwide. He holds two patents on non-invasive biomarkers of NAFLD and fibrosis.
His research is funded by the National Institutes of Health including R01, U01, P30 and P01 grant mechanisms, Foundation of NIH, National Science Foundation as well as several investigator initiated research projects funded by the industry. He is the Principal Investigator, UCSD, for the NIDDK-sponsored NASH Clinical Research Network. He served as the elected Chair of the NAFLD, Special Interest Group of the American Association for the Study of Liver Diseases. And is the elected member to the National Board of Directors of the American Liver Foundation.
He serves on the Editorial Board of Gastroenterology, Journal of Hepatology, GUT and Nature Reviews in Gastroenterology and Hepatology. He is the Deputy Editor of HEPATOLOGY, the official journal of the AASLD.
Dr. Loomba has published more than 250 manuscripts and has an H-index of 76. He is among the top 1% of the globally highly cited scientists across all fields in 2019 by Web of Science. He is an elected member of American Society of Clinical Investigation.
Mark McNiven, PhD
Professor of Biochemistry and Molecular Biology, Mayo Clinic
Mark A. McNiven, Ph.D., is a consultant in the Division of Gastroenterology and Hepatology, Department of Internal Medicine, with a joint appointment in the Department of Biochemistry and Molecular Biology at Mayo Clinic in Rochester, Minnesota. Dr. McNiven is director of the Gastrointestinal Cancer Program at Mayo Clinic Cancer Center and director of the Mayo Center for Biomedical Discovery. He holds the academic rank of Professor of Biochemistry and Molecular Biology, Mayo Clinic College of Medicine and Science, and is recognized with the distinction of the George M. Eisenberg Professorship.
Dr. McNiven attended State University of New York for his undergraduate degree and the University of Colorado for his graduate studies. He earned his Ph.D. at the University of Maryland, where he also completed postdoctoral studies in cell biology. He further completed postdoctoral training in the Department of Cell Biology and Anatomy at Johns Hopkins University School of Medicine.
Dr. McNiven's laboratory focuses on the molecular basis by which tumor cells grow unchecked and migrate from the primary organ to invade and metastasize into distal sites. In addition, his program studies the cellular basis by which cells of the liver can become laden with fat leading to steatohepatitis, metabolic diseases and cancer. He is the principal investigator of the first Hepatobiliary Cancer SPORE (Specialized Program of Research Excellence) in the country and also the PI of a new NIDDK pre-doctoral training grant.
His research is funded by the National Cancer Institute, the National Institute on Alcohol Abuse and Alcoholism, and the National Institute of Diabetes and Digestive and Kidney Diseases. His work has been published in prominent peer-reviewed journals, including the Journal of Cell Biology, Developmental Cell, Nature Cell Biology, Hepatology, Nature Genetics, Trends in Cellular Biology, Molecular Biology of the Cell, Gastroenterology, Cancer Research, and Science.
James Topper, MD, PhD
Frazier Healthcare Partners
Jamie co-leads Frazier's Life Sciences team and has over 25 years of experience working with entrepreneurs to found and build successful therapeutics-focused companies.
Jamie is a Managing Partner of Frazier Healthcare Partners' Life Sciences team. He joined Frazier in 2003 and opened Frazier's Menlo Park office in the same year. Throughout his 15 years as a Managing Partner, Jamie has invested across over 35 companies encompassing a broad spectrum of Life Science and Biopharmaceutical companies.
Prior to joining Frazier, Jamie was the head of cardiovascular R&D at Millennium Pharmaceuticals and ran Millennium San Francisco (formerly COR Therapeutics). Before the merger of COR and Millennium, he served as the Vice President of Biology at COR and was responsible for all research activities. He served on the medical school faculties at Stanford and Harvard Medical School prior to joining COR.
Jamie received his MD and PhD in Biophysics from Stanford and holds a BS from the University of Michigan. He did his postgraduate training in Internal Medicine and Cardiovascular Disease at the Brigham and Women's Hospital in Boston. He has authored over 50 publications and was the recipient of a Howard Hughes Scholars Award while on the faculty at Stanford.
In 2011 and 2016 Jamie was named to the Midas List of leading venture capitalists, and in 2013 was recognized by Forbes as one of the top 10 healthcare investors.
Roger Ulrich, PhD
Former CSO, Acerta
Roger has several decades of both leadership and operational experience within the pharmaceutical industry and is a Senior Advisor on the Frazier Life Sciences team.
Roger was with Acerta Pharma as both a member of the company's founding Board of Directors and as Chief Scientific Officer through its majority-share acquisition by AstraZeneca. He was previously co-founder and Chief Development Officer with Calistoga Pharmaceuticals and guided the development of CAL-101 (GS1101, idelalisib), an isoform-selective PI3K inhibitor. Calistoga was acquired by Gilead Sciences, and Roger continued with Gilead to help with the Calistoga integration and complete the development of CAL-101, now marketed as Zydelig.
Prior to Calistoga, Roger was Senior Scientific Director with Merck Research Laboratories, where he helped guide the Merck integration of Seattle-based Rosetta Inpharmatics. Prior to Merck, he was Director of Drug Safety Evaluation at Abbott Laboratories, and was Senior Scientist with both The Upjohn Company and Pharmacia & Upjohn Inc. Roger directly contributed to the discovery, development, approval, and life cycle of several products, including Zydelig, Camptosar, Zyvox, Spexil, Colestid Tablets, Actos, Kaletra and Humira. Prior to his career in the pharmaceutical industry, he held research positions at West Virginia and Michigan State Universities, and at Argonne National Laboratories.
Roger received his PhD in Cellular and Molecular Biology from West Virginia University, where he studied the role of protein kinases and cytoskeletal function in cancer. He holds several patents, has authored more than 120 publications, and is a Fellow of the Academy of Toxicological Sciences.
news and publications
- November 30, 2023 Inipharm Initiates Dosing in Phase 1 Study of Its Small Molecule Inhibitor of HSD17B13
- June 7, 2023 Inipharm’s Development Candidate INI-822 Shows Improvements in Markers of Liver Homeostasis in Preclinical Studies
- October 25, 2022 Inipharm to Present Data on Anti-Fibrotic Effects of Its Development Candidate Targeting HSD17B13 at AASLD’s The Liver Meeting
- June 8, 2022 Inipharm Appoints Chuhan Chung, M.D, as Chief Medical Officer to Advance HSD17B13 Program into the Clinic
- October 05, 2021 Inipharm to Present Data Showing Potential of Small Molecule Inhibitors of HSD17B13 to Combat Liver Fibrosis at AASLD's The Liver Meeting
- November 11, 2020 Inipharm Raises $35 Million in Series A to Focus on Highly Validated Genetic Target HSD17B13


